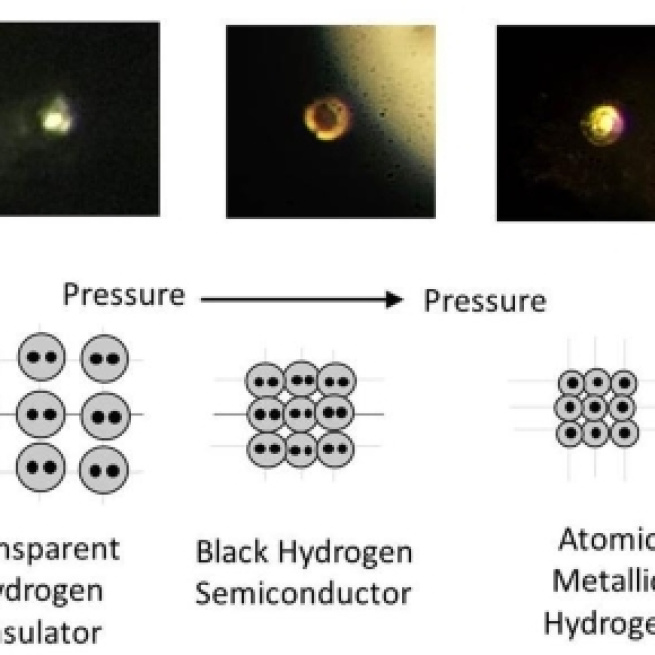
Meet hydrogen, the metal
Hydrogen is the most abundant element in the earth’s atmosphere where it exists in the gaseous phase. In the 1930s it was theoretically predicted by Eugene Wigner and Hiddard Bell that hydrogen could exist in a conductive metallic state under certain conditions. They calculated the energy and electronic conduction of a body centre cubic lattice of hydrogen and found the minimum in energy (the most stable state) corresponds to a lattice spacing of an extraordinarily high density material. The hypothesis remained untested for a century due to the immense high pressure needed to produce the material from regular hydrogen (25 GPa - 25 million times greater than the standard atmospheric pressure on Earth).
In January 2017, researchers Isaac F. Silva and Ranga P. Dias working at harvard university announced that they had successfully observed the metallization of hydrogen at a pressure of 465 GPa and temperature of 5.5K using a diamond anvil. In a feat analogous modern-day alchemy, the group had claimed to produce a tiny amount of rare Metallic Hydrogen which was seen to be highly reflective – the first of its kind to ever exist on Earth. The original findings were released on the open source journal arXiv and in February the work was published in the prestigious journal Science (paper titled: Observation of the Wigner-Huntington transition to metallic hydrogen).
The hype
Aside from the fundamental intrigue which is natural when a new exotic state of matter is discovered, the material may have a wide array of useful applications.
For example, metallic hydrogen could be the key to achieving room temperature superconductivity which was predicted by physicist Neil Ashcroft in the 1960s.
It is an interesting time for electronics, with scientists pushing the boundaries with exotic nanotechnology and molecular electronics, such as using DNA molecules as electronic switches by modifying the molecular structure and linking to an anthraquinone (Aq) molecule and enabling regulation of #Electron flow. As for metallic hydrogen, a more realistic application could be as a potent rocket fuel for interstellar exploration due to the large amount of energy stored in the high density material. To quote the original authors: “Metallic hydrogen may be a room-temperature superconductor and metastable when the pressure is released and could have an important impact on energy and rocketry.”
The fallout
The findings have not been widely accepted by academic community, indeed the work has broadly been met with a healthy dose of cynicism.
Mikail Eremets from the Max Planck Institute in Mainz who is also working on high pressure hydrogen studies, has been outspoken about his scepticism of the methods of Silva and Dias. "They might be lucky" Eremets said, speaking to Chemistry World. "They used good diamonds, maybe it helped. But we’ve done the same for three years at least. In our experience, it’s impossible to reach such high pressures." Other researchers have been more positive on the outlook, but have been keen to stress that the claims have to stand up to severe scrutiny and testing.
The authors have since posted a response to the criticisms in a post print paper on arXiv in which they sought to discuss some of the concerns raised by the scientific community.
In the paper, they justify their calculations of pressure, while admitting there may be 'large systematic uncertainties' which are common in high pressure physics, but concluding with the bullish reaffirmation: 'There is no doubt that metallic hydrogen was produced at the highest pressures.’
It has been revealed that further analysis of the original sample used for the original publication is now impossible, because in testing the diamond used in the experiment shattered under high pressure, rendering the precious, and highly debated, metallic hydrogen unusable.